Dr. Hootan Daneshmand
Breast Implant Removal - Bakersfield, CA
Home » Our Procedures » Breast Surgery Procedures » Breast Implant Removal
Explant Surgery
Breast augmentation remains the most popular cosmetic surgery procedure in the United States. So, it stands to reason that out of this large group, a small percentage of women (or men) choose to undergo breast implant removal surgery, or explant surgery. In some cases, the breast implants no longer suited the patient’s lifestyle, and in other cases, the breast implants needed to be removed for health reasons.
With recent news regarding certain implants being associated with very rare forms of cancer, such as BIA-ALCL and BIA-SCC, as well as some patients reporting they feel that their implants are causing systematic health issues, more women are starting to ask if they should have their breast implants removed.
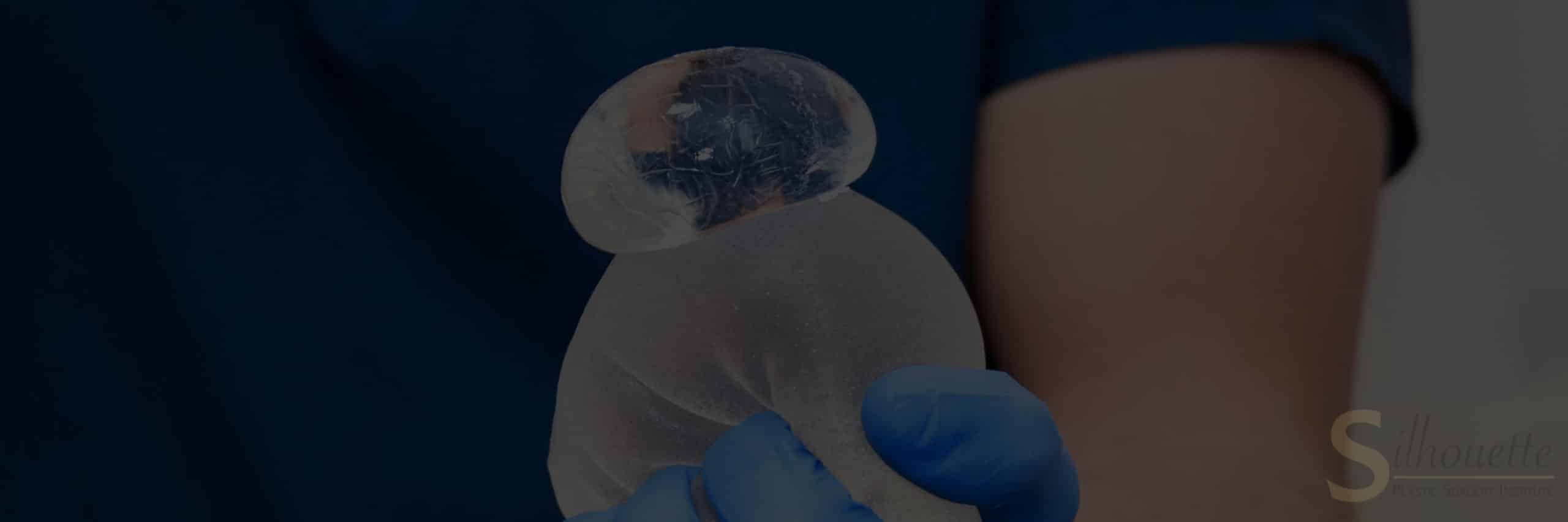
Should I Have My Breast Implants Removed?
Whether or not to have your breast implants removed for health reasons is a personal decision that you should make alongside your doctor. The vast majority of breast augmentation patients remain very happy with their implants. However, a small number of patients who have explanation surgery are also happy with their decision.
What are the Reasons for Breast Implant Removal?
While every person's reason for having his or her breast implants removed are personal, here are the most common reasons for having explant surgery:
- personal preference
- capsular contracture
- implant rupture
- a desire to rule out implants as a source of health problems
- medical necessity
Breast implant removal is an appropriate option as long as it is what the patient wants. The surgery can be performed safely. If you do want to have your breast implants removed, you'll want to choose a board certified plastic surgeon who has experience in this procedure.
You may also want to look into breast implant revision surgery, or breast reconstruction.
What is Breast Implant Removal Surgery?
Breast implant removal surgery, or explanation, is typically performed as an outpatient procedure. We use general anesthesia, although in some cases, local anesthesia can be used to remove saline implants.
Whenever possible, we use the same incision site used for the initial breast augmentation procedure. Through this incision, we carefully remove the implant and the surrounding scar tissue, then close the incisions.
What is Breast Implant Removal Recovery Like?
You typically need less downtime after explanation than you did after your breast augmentation. For most patients, 5 days off is plenty of time. However, you may want to refrain from strenuous exercise and lifting for a few weeks, just until your incisions heal.
What Will My Breasts Look Like After Explantation?
Your breasts will be smaller, and depending on how long you've had your implants, how large the implants were compared to your natural breasts' size, whether or not you've had children, your breasts may look similar to or very different from before your augmentation.
It's important to keep in mind that having implants doesn't freeze the aging process, nor does it prevent other tissue changes.
Immediately after surgery, you can expect some swelling, bruising and soreness, just like you did after your breast augmentation.
Your breast implant removal scars will be present, although they should fade over time.
Do I Need En Bloc Capsulectomy?
This is a specific explantation technique that involves removing the breast implant and surrounding capsule of scar tissue as one single piece. With this technique, the capsule is separated from the surrounding breast tissue without breaking it. It's then removed with the implant still sealed inside.
There are a few benefits for en bloc capsulectomy. This certainly holds true if we're removing a ruptured silicone implant. Keeping the capsule intact ensures that the silicone is contained. We'll also recommend an en bloc capsulectomy if a complication, such as BIA-ALCL is suspected.
However, the en bloc capsulectomy technique does not come without its drawbacks. First, a much larger incision is required. We cannot collapse or squeeze the implant or capsule during the removal. Second, it's not always a safe or practical option. This especially holds true if the capsule is very thin or is closely adhered to the chest tissue.
The en bloc capsulectomy technique is not the only one that allows for complete removal of the capsule and breast implant. For most patients, it's better to open the capsule, carefully remove the implant, close it, and then go back and release the capsule from the surrounding breast tissue before removing it.
Breast Implant Illness
If you are considering explantation because you are worried about or think you may have breast implant illness, we understand your fear. A number of patients who have had their breast implants removed say symptoms such as fatigue, joint pain, or headaches improved or they felt better, in general, afterwards. Other patients didn’t experience any improvement, but still felt better to have eliminated a source or concern for them, personally.
Its important to note that there is no scientific research proving that breast implants can cause systemic issues. Therefore, we cannot guarantee that removing your implants will improve your health. Be sure to contact your primary care doctor first to rule out any diagnosable causes for symptoms, such as Lyme disease.
If the patients wants his or her breast implants removed and is a good candidate for surgery, then we will work to provide the most aesthetically pleasing result possible.
If you would like to discuss your concerns and procedure options further, contact Silhouette Plastic Surgery to schedule a consultation.
Anaplastic Large Cell Lymphoma (ALCL)
In 2011, the FDA identified a possible association between breast implants and the development of anaplastic large cell lymphoma (ALCL), a rare type of non-Hodgkin’s lymphoma. At that time, the Agency was aware of approximately 60 cases of ALCL in women with breast implants, out of approximately 5-10 million women who had received breast implants worldwide. This included 34 unique cases that were described in the medical literature from January 1, 1997, through May 21, 2010, and additional cases identified by international regulatory agencies, scientific experts, and breast implant manufacturers. Based on this data, the FDA cautioned patients and health care providers that women with breast implants might have a very low but increased risk of developing ALCL.
The FDA also posted on this website a detailed analysis of the 34 cases of ALCL in women with breast implants, provided physicians with interim recommendations on identifying and confirming ALCL, and worked with breast implant manufacturers to include information regarding ALCL in the patient and physician labeling.
Because the FDA knew of so few cases of this disease, it was impossible to determine what factors increased the risk. In a report summarizing the Agency’s findings, we emphasized the need to gather additional information to better characterize ALCL in women with breast implants.
